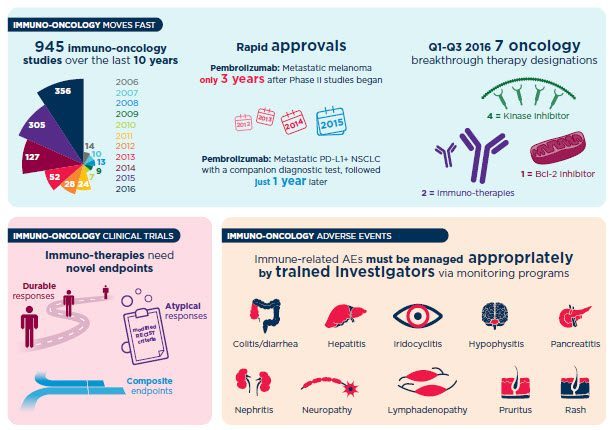
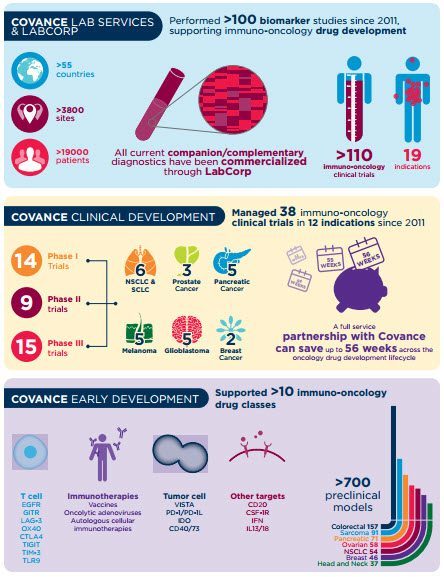
Complex immuno-oncology drug development requires innovation, commitment and expertise.
Precision medicine. Advanced diagnostics.
Our industry’s deeper understanding of the immune system’s involvement in cancer has uncovered new approaches in the treatment of cancer. You need to quickly screen and identify safe and efficacious molecules, both alone and in combination, to quickly advance to the next milestone. Leverage our deep science expertise and experience in precision medicine to support your testing needs and execute on innovative trial designs that allow for the success of your unique therapy.
Whether helping you find the best compounds for combination therapy, identifying and stratifying biomarkers, developing companion diagnostics or revealing your market opportunities, our IO experience and global clinical CRO capabilities can deliver solutions from discovery through clinical trials and post-approval activities to advance your development at any stage.