Supporting Your Assay and Biomarker Needs for COVID-19
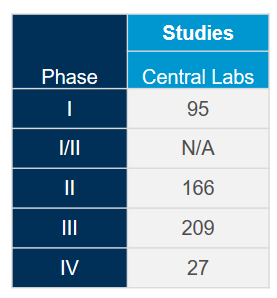
Extract the most value out of our globally integrated central laboratory network with the help of our scientific and operational experts. With deep experience gained from more than 4,400 clinical trials during the past five years across all therapeutic areas, we know how to optimize your studies and mitigate potential issues to meet the fast-pace associated with coronavirus (COVID-19) studies.