Author: Chris Bull, Sr. Scientific Advisor, Imaging
Date: January 2016
Glioblastomas are known to have a poor prognosis with median survival of nine months and only five to 10 percent of patients surviving up to two years. Conventional therapies include radiotherapies and surgical removal of the tumor in combination with chemotherapy.
Unfortunately, these tumors can often be radioresistant and surgical removal of the entire tumor may not be possible. Glioblastoma (GBM) is a highly complex disease with little effective treatment options. New Immunotherapy drugs provide an alternate approach to treatment, aiming to bolster the immune system to eradicate disease. In the past, use of antibody therapies has been limited in GBM due to the obstruction caused by the blood brain barrier to therapy delivery. However investigation of immuno-oncology drugs may overcome this limitation as one may only need the immune cells to cross the blood brain barrier. Preclinical testing of this class of drugs requires the use of a unique set of preclinical models utilizing immunocompetent mice and orthotopic placement of the tumor cells within the brain. We have the technical capability to perform over 100 orthotopic GBM procedures a day.
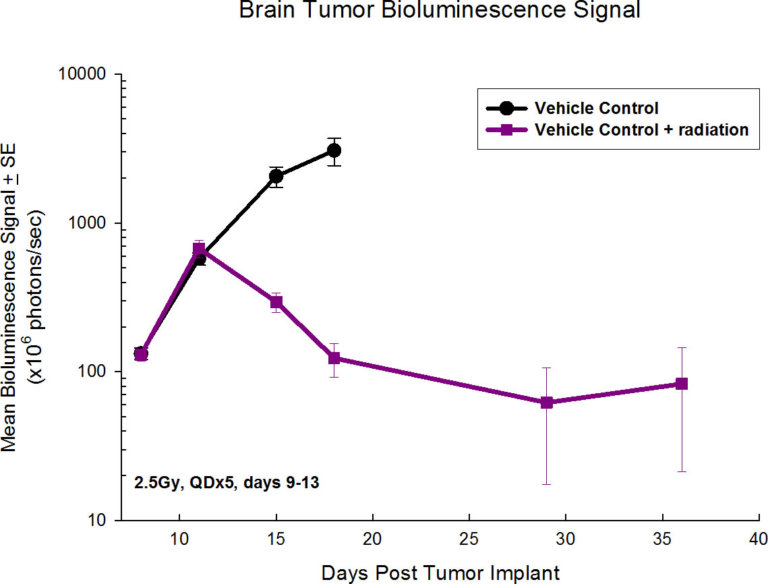
GL261 is one of the most frequently used syngeneic murine glioma models available. We capitalize on our state-of-the-art imaging capabilities to further interrogate the pharmacology of this model by utilizing a GL261 cell line that expresses luciferase (GL261-luc2, Caliper Life Sciences). This allows for longitudinal, in vivo visualization of the tumors growth and response to treatment within the brain via bioluminescence.
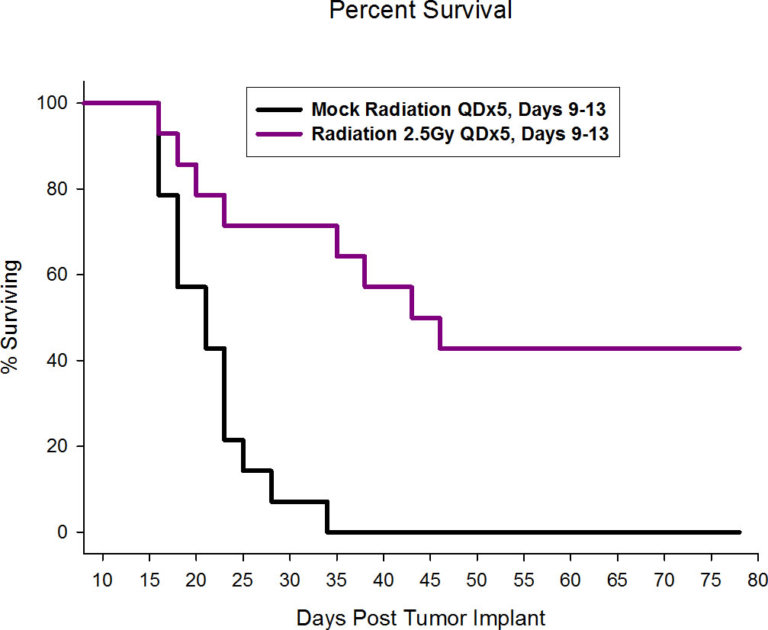
At Labcorp, we have the ability for shielded focal radiation delivery to the brain, and more recently we have acquired the Xstrahl Small Animal Radiation Research Platform (SARRP) allowing for pinpoint irradiation of only the tumor sparing the rest of the mouse, including sites critical for mounting an immune response. While overall survival is a common endpoint in preclinical GBM studies, the addition of in vivo imaging endpoints can add further value to the overall study dataset.
In addition to bioluminescence, we have a host of other imaging technologies, including PET, SPECT and CT modalities, to further interrogate parameters related to GBM such as tumor volume, vascular perfusion/permeability, metabolic activity, and edema.
At Labcorp, we offer a combination of multiple syngeneic tumor lines along with in vivo imaging, guided irradiation treatment, and flow cytometry to best evaluate novel immunotherapies. Furthermore, high throughput capabilities allow rapid and automated acquisition of large sample sizes to accommodate the growing complexity of our client’s preclinical studies.
Contact us to design your GL261 study today!
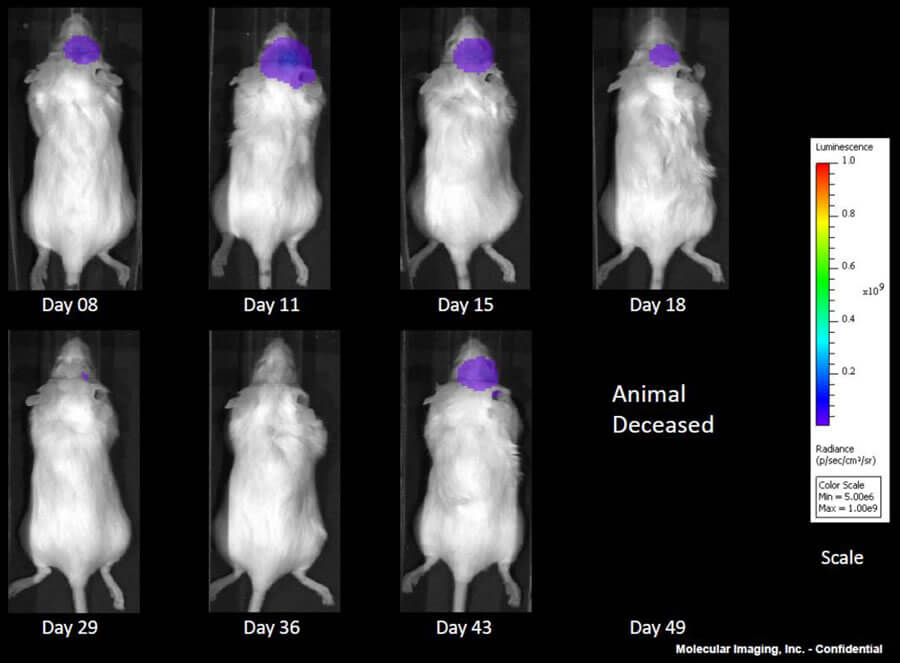
Note: Please note that all animal care and use was conducted according to animal welfare regulations in an AAALAC-accredited facility with IACUC protocol review and approval.
Connect
Let's start a conversation
Contact Us